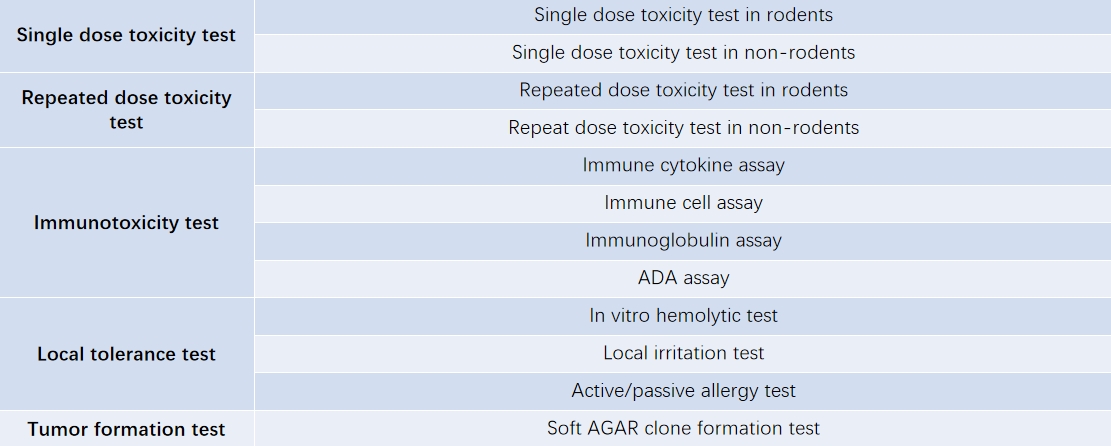
Non-GLP (non-good laboratory practice) toxicology studies are usually conducted in the early drug discovery and preliminary toxicology evaluation stages, with the purpose of examining the toxicological properties of new compounds to guide further study design and project direction. At present, CIR Biopharma has established a pre-toxicology platform for early drug discovery and development, which can provide customers with services such as single-dose toxicity testing, repeated-dose toxicity testing, immunotoxicity testing, local tolerance testing and tumorigenicity testing.