Humanized tumor model
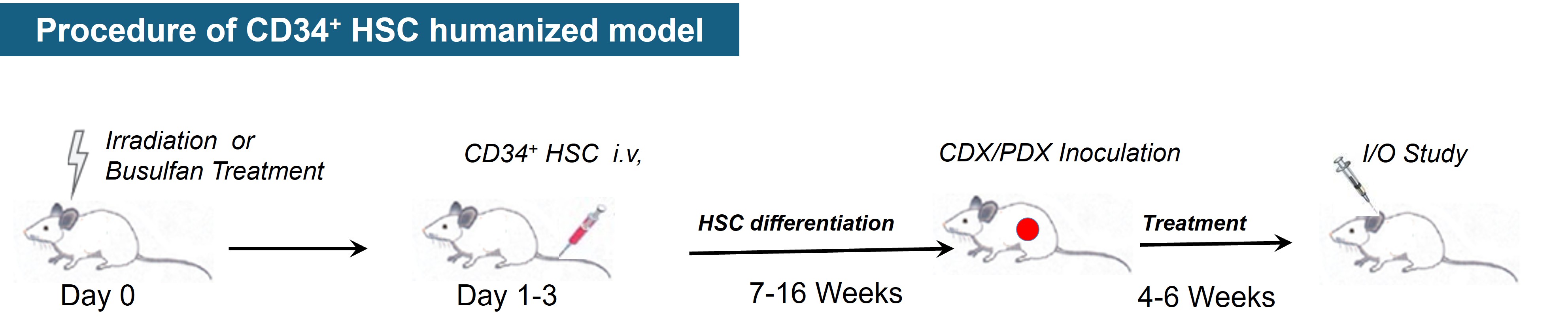
hHSC humanized tumor model
The hHSC (humanized-hematopoietic stem cells) humanized tumor model is a method of injecting human CD34+ HSCs into immunodeficient host mice, which involves first irradiating the host mice with a sublethal dose of irradiation in order to eliminate the mouse HSCs and to facilitate the engraftment of human HSCs. hHSCs are a group of cells in human hematopoietic tissues that can self-renew and differentiate to produce various lineages of hematopoietic cells. The CD34 antigen, which is widely recognized as a representative surface marker of hematopoietic stem/progenitor cells, is a highly glycosylated type I transmembrane glycoprotein that regulates cell adhesion and promotes adhesion to the bone marrow stroma. Injection of human CD34+ HSCs from human umbilical cord blood, bone marrow, G-CSF-activated peripheral blood, or fetal liver into adult immunodeficient mice by intravenous (i.v.) or intra-femur (i.f.) injection produces a wide range of hematopoietic stem cells, but low T-cell production and no functional immune cells. Alternatively, transplantation of human CD34+ HSCs into neonatal recipient mice (less than 4 weeks of age) by intravenous injection (intracardiac or intrahepatic) yielded good human cell engraftment and production of T cells, B cells, macrophages, NK cells, and DCs.
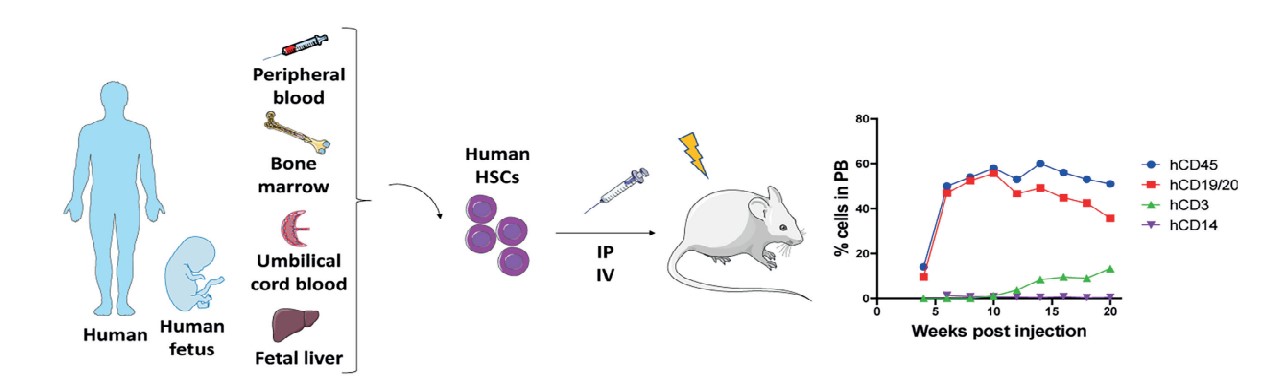
Cogels M, et al. Front Oncol. 2021, 11:784947.
hHSC humanized tumor models for drug evaluation
hPBMC humanized tumor model
Humanized-peripheral blood mononuclear cells (hPBMC) humanized mouse tumor model is a method by injecting human PBMCs intravenously into severely immunodeficient mice. The human immune system is partially reestablished in the peripheral blood and lymphoid tissues of the mice, and the number of hPBMCs injected into mice generally ranges from 5-10x106. 3-4 weeks after hPBMC injection, high levels of human immune cells, predominantly hCD45+CD3+ T-cells, with lower levels of human B-cells and myeloid cells, could be detected in the peripheral blood of mice. Although transplanted hCD45+CD3+ T cells eventually react with mouse major histocompatibility complex (MHC) class I and II molecules, inducing graft-versus-host disease (GvHD), resulting in the death of the mice within 6-8 weeks after successful immune reconstitution. However, due to the advantages of easy preparation, short cycle time, high levels of hCD45+CD3+ T cells in the peripheral blood of mice, and the possibility of transplanting hPBMCs and tumor tissues from the same patient, the hPBMCs model has been sought after and is widely used.
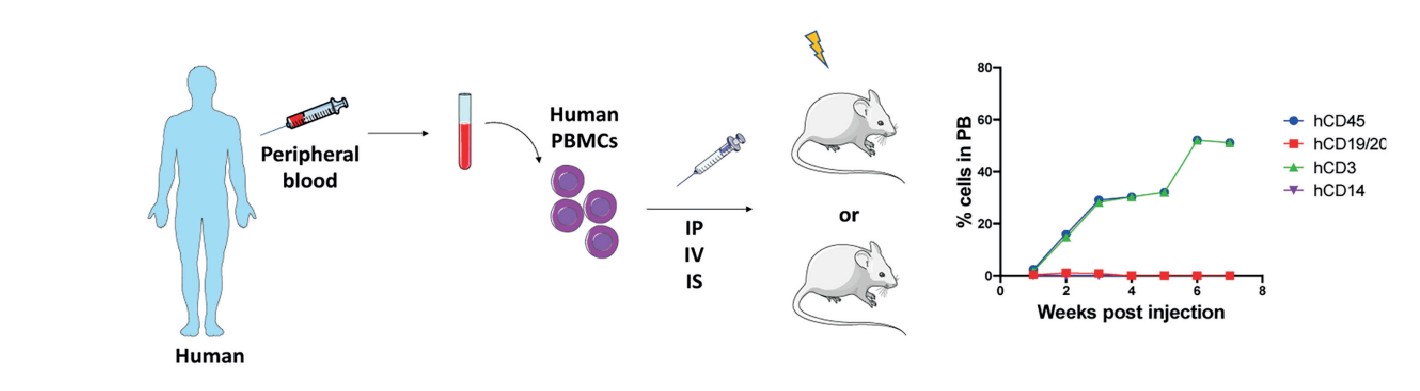
Cogels M, et al. Front Oncol. 2021, 11:784947.
hPBMC humanized tumor models for efficacy evaluation
hNK cell humanized tumor model
Natural killer (NK) cells account for about 5-20% of the total lymphocytes and can respond specifically to viruses or malignant cells. When detecting viral infections or tumor cells, they target and destroy them by inducing apoptosis, and at the same time send out danger signals through the release of inflammatory cytokines, so that NK cells play an important role in the removal of cancer cells and cancer therapy. NK cells are capable of directly killing virus-infected cells and tumor cells without prior activation, not only rapidly recognizing and exerting direct killing of target cells in the early stages of tumorigenesis, but also engaging with immune cells, including DCs, to activate adaptive immune responses to enhance CD8+ T cell-mediated antitumor responses. In a variety of tumor settings, the antitumor capacity of CD8+ T cells requires NK cell function, which expresses a variety of activating receptors, inhibitory receptors, and cytokine or chemokine receptors on their surface, and whose activation and function depend on a balance between signaling from inhibitory receptors and activating receptors. Currently, it is commonly used to use an animal model of humanization of the immune system to obtain mice implanted with the human immune system, inoculated with tumor cells and then administered for treatment. CIR Biopharma has developed such an animal model to meet the various needs for the development of NK cell-related therapeutic drugs.
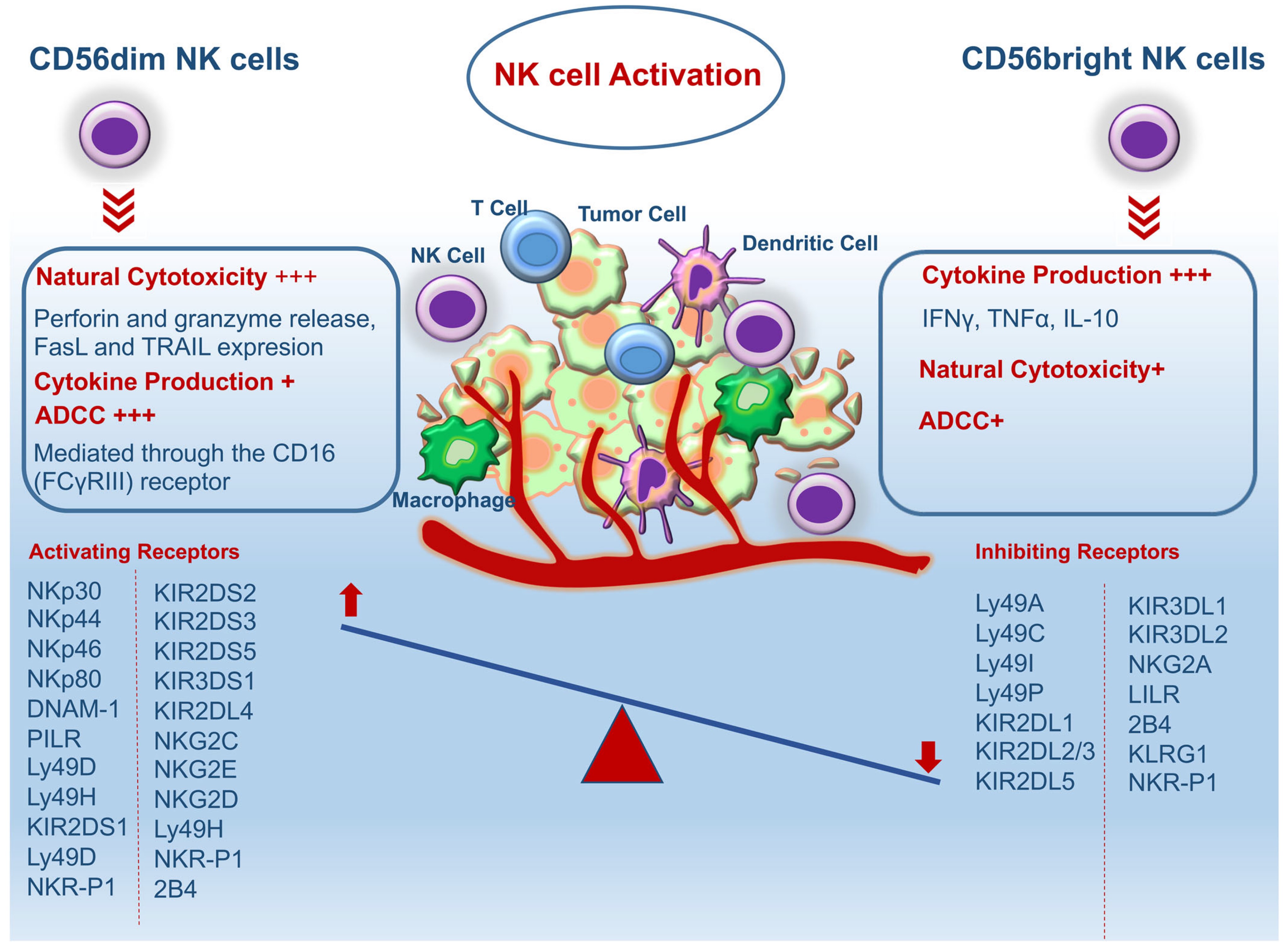
Schematic diagram of types and functions of NK cells for killing tumors.
Ghaedrahmati F, et al. Cancer Commun (Lond). 2023; 43(2):177-213.
hM myeloid cell humanized tumor model
CIR Biopharma has successfully developed myeloid immune cell humanized tumor models to provide our clients with more technical services for hM myeloid cell therapeutic drug development. Myeloid cells are important immune cells in the tumor microenvironment and can play both immunosuppressive and immunostimulatory roles in the tumor microenvironment. Myeloid cells include many different cell types, such as monocytes, macrophages, and DCs, which are highly plastic and can differentiate into different phenotypes depending on the characteristics of the microenvironment. The development of humanized reconstructive tumor models for myeloid immune cells can help the mining of corresponding targets and the development of therapeutic strategies.
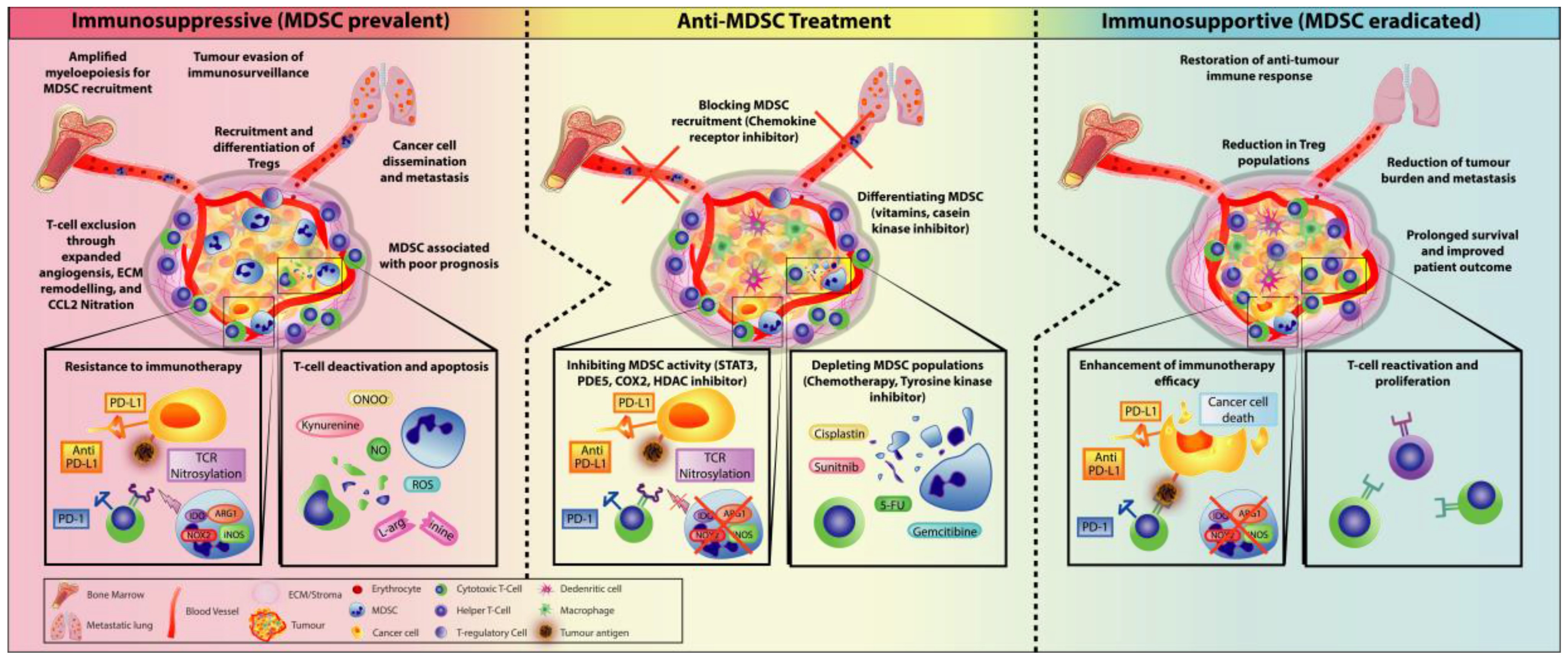
Law A, et al., Cells. 2020; 9(3):561.